Introduction
Introduction
Q. What is Biomolecule?
A biomolecule (biological molecule) is any molecule that is present in living organisms – microorganisms, plants and animals. They are mostly made up of carbon, oxygen, hydrogen and nitrogen.
 Key Points
Key Points
- Proteins, carbohydrates, lipids, and nucleic acids [DNA and RNA] are Macromolecules or Macro-biomolecules.
- Other small molecules such as vitamins, primary metabolites, secondary metabolites, etc. are also biomolecules.
- Most biomolecules are organic compounds.
Note: Metabolism = the chemical processes that occur within a living organism to maintain life.
Metabolite = a substance formed in or necessary for metabolism.
Primary metabolite = Metabolite that is directly involved in normal growth, development, and reproduction. Eg: ethanol, lactic acid, and certain amino acids.
Secondary metabolite = Metabolites that are not directly involved in the normal growth, development, or reproduction of an organism. Unlike primary metabolites, absence of secondary metabolites does not result in immediate death, but rather in long-term impairment. Eg: ergot alkaloids, antibiotics, etc.
Alkaloid = any of a class of nitrogenous organic compounds of plant origin which have pronounced physiological actions on humans.
Example: morphine obtained from opium poppy.
 Biomolecules
Biomolecules
- Carbohydrates are one of the most important biomolecules that forms a major part of the living things.
- Carbohydrates are primarily produced by plants and form a very large group of naturally occurring organic compounds.
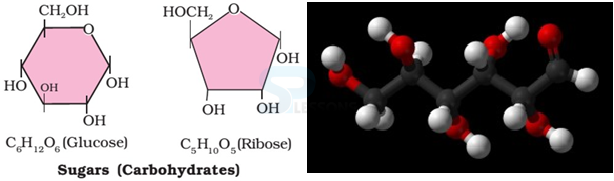
- Some common examples of carbohydrates are cane sugar, glucose, starch
- Most of them have a general formula, Cx(H2O)y, and were considered as hydrates of carbon from where the name carbohydrate was derived.
- For example, the molecular formula of glucose (C6H12O6) fits into this general formula, C6(H2O)6. But all the compounds which fit into this formula may not be classified as carbohydrates. Acetic acid (CH3COOH) fits into this general formula Cx(H2O)y → C2(H2O)2 but is not a carbohydrate. Exception: Rhamnose, C6H12O5 is a carbohydrate but does not fit in this definition of Cx(H2O)y.
- Chemically, the carbohydrates may be defined as optically active polyhydroxy [multiple HO groups] aldehydes or ketones or the compounds which produce such units on hydrolysis. Carbohydrates produce aldehydes and ketones on hydrolysis [the chemical breakdown of a compound due to reaction with water].
- Some of the carbohydrates, which are sweet in taste, are also called sugars.
- The most common sugar, used in our homes is named as sucrose whereas the sugar present in milk is known as lactose.
- Carbohydrates are also called saccharides (Greek: sakcharon means sugar).
- Carbohydrates are classified on the basis of their behavior on hydrolysis. They have been broadly divided into following three groups.
Image: Carbohydrates
Source: NCERT Text Books
Hydrate = a compound in which water molecules are chemically bound to another compound or an element. Eg: α-d-Glucose hydrate (C6H14O7). Note:
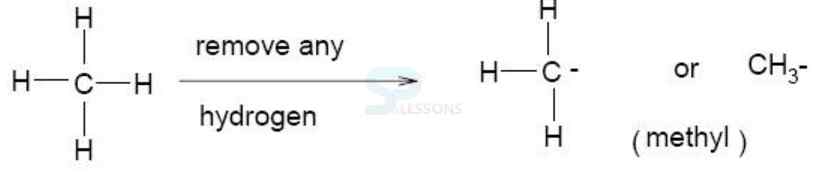
Aldehyde = an organic compound containing the group — CHO, formed by the oxidation of alcohols. Typical aldehydes include methanal (formaldehyde) and ethanal (acetaldehyde).
Ketone = an organic compound containing a carbonyl group = C = O bonded to two alkyl groups, e.g. acetone].
Alkyl = denoting a hydrocarbon radical derived from an alkane by removal of a hydrogen atom].
Alkane = any of the series of saturated hydrocarbons including methane, ethane, propane, and higher members].
Image: alkane-alkyl
Source: NCERT Text Books
- A carbohydrate that cannot be hydrolyzed further to give simpler unit of polyhydroxy aldehyde or ketone is called a monosaccharide.
- About 20 monosaccharides are known to occur in nature. Some common examples are Glucose, Fructose, Ribose, Galactose, etc.
- If a monosaccharide contains an aldehyde group [–CHO], it is known as an aldose and if it contains a keto group [=C=O], it is known as a ketose.
- Glucose occurs freely in nature as well as in the combined form.
- It is present in sweet fruits and honey. Ripe grapes also contain glucose in large amounts.
- Glucose is an aldohexose [An aldohexose is a hexose with an aldehyde group on one end] and is also known as dextrose. It is the monomer of many of the larger carbohydrates, namely starch, cellulose. Aldohexose == An aldohexose is a hexose with an aldehyde group on one end. Aldehyde group [–CHO] Hexose == any of the class of simple sugars whose molecules contain six carbon atoms (e.g. glucose)
- It is probably the most abundant organic compound on earth.
- Glucose is found to exist in two different crystalline forms which are named as [latex]\alpha[/latex] and [latex]\beta[/latex].
- Such isomers, i.e., α-form and [latex]\beta[/latex]-form, are called anomers.
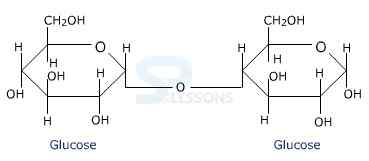
- Fructose is an important ketohexose. It is obtained along with glucose by the hydrolysis of disaccharide, sucrose.
- The two monosaccharides are joined together by an oxide linkage formed by the loss of a water molecule.
- Such a linkage between two monosaccharide units through oxygen atom is called Glycosidic Linkage.
Image: Glycosidic Linkage
Source: NCERT Text Books
Ribose- The ribose [latex]\beta[/latex]-D-ribofuranose forms part of the backbone of RNA. It is related to deoxyribose, which is found in DNA. Galactose
- Galactose is a monosaccharide. When combined with glucose (monosaccharide), through a condensation reaction, the result is the disaccharide lactose.
- The hydrolysis of lactose to glucose and galactose is catalyzed by the enzymes lactase and [latex]\beta[/latex]-galactosidase.
- Carbohydrates that yield two to ten monosaccharide units, on hydrolysis, are called oligosaccharides.
- They are further classified as disaccharides, trisaccharides, tetrasaccharides, etc., depending upon the number of monosaccharides, they provide on hydrolysis.
- Amongst these the most common are disaccharides.
- The two monosaccharide units obtained on hydrolysis of a disaccharide may be same or different.
- For example, sucrose on hydrolysis gives one molecule each of glucose and fructose whereas maltose gives two molecules of glucose only.
Note:Sucrose == Glucose + Fructose
Maltose == Glucose + Glucose
Lactose == Glucose + Galactose
- One of the common disaccharides is sucrose which on hydrolysis gives equimolar mixture of glucose and fructose.
- Another disaccharide, maltose is composed of two α-D-glucose units
- It is more commonly known as milk sugar since this disaccharide is found in milk. It is composed of β-D-galactose and β-D-glucose.
- Carbohydrates which yield a large number of monosaccharide units on hydrolysis are called polysaccharides.
- Some common examples are Starch, Cellulose, Glycogen, Gums.
- Polysaccharides are long chains of sugars. Polysaccharides are not sweet in taste, hence they are also called non-sugars.
- They are threads (literally a cotton thread) containing different monosaccharides as building blocks.
- For example, Cellulose is a polymeric polysaccharide consisting of only one type of monosaccharide i.e., Glucose. Cellulose is a homopolymer. Starch is a variant of this but present as a store house of energy in plant tissues.
- Animals have another variant called Glycogen.
- Inulin is a polymer of fructose.
- Plant cell walls are made of cellulose. Paper made from plant pulp and cotton fibre is cellulosic. There are more complex polysaccharides in nature.
- Exoskeletons of arthropods, for example, have a complex polysaccharide called These complex polysaccharides are mostly homopolymers.
- Polysaccharides contain a large number of monosaccharide units joined together by glycosidic linkages.
- These are the most commonly encountered carbohydrates in nature.
- They mainly act as the food storage or structural materials.
- Starch is the main storage polysaccharide of plants.
- It is the most important dietary source for human beings.
- High content of starch is found in cereals, roots, tubers and some vegetables.
- It is a polymer of α-glucose and consists of two components — Amylose and Amylopectin.
- Amylose is water soluble polysaccharide which constitutes about 15-20% of starch.
- Amylopectin is water insoluble polysaccharide which constitutes about 80- 85% of starch.
- Cellulose occurs exclusively in plants and it is the most abundant organic substance in plant kingdom.
- It is a predominant constituent of cell wall of plant cells.
- Cellulose is a straight chain polysaccharide composed only of β-D-glucose units.
- The carbohydrates are stored in animal body as
- It is also known as animal starch because its structure is similar to amylopectin and is rather more highly branched.
- It is present in liver, muscles and brain.
- Glycogen is also found in yeast and fungi.
- When the body needs glucose, enzymes break the glycogen down to glucose.
- Carbohydrates are essential for life in both plants and animals.
- They form a major portion of our food. Honey has been used for a long time as an instant source of energy in ayurvedic system of medicine.
- Carbohydrates are used as storage molecules as starch in plants and glycogen in animals.
- Cell wall of bacteria and plants is made up of cellulose which is a carbohydrate.
- We build furniture, etc. from cellulose in the form of wood and clothe ourselves with cellulose in the form of cotton fibre.
- They provide raw materials for many important industries like textiles, paper, lacquers and breweries.
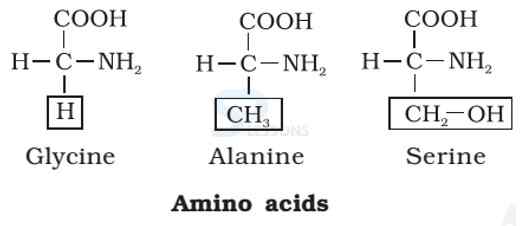
- Amino acids are organic compounds containing an amino group [NH2] and an acidic group [COOH] as substituents on the same carbon i.e., the a-carbon. Hence, they are called a-amino acids. They are substituted methanes.
- All proteins are polymers of α-amino acids.
- Amino acids contain amino (–NH2) and carboxyl (–COOH) functional groups.
- Depending upon the relative position of amino group with respect to carboxyl group, the amino acids can be classified as [latex]\alpha[/latex], [latex]\beta[/latex], [latex]\gamma[/latex], [latex]\delta[/latex] and so on.
- Only α-amino acids are obtained on hydrolysis of proteins.
- All α-amino acids have trivial names, which usually reflect the property of that compound or its source.
- Glycine is so named since it has sweet taste (in Greek glykos means sweet) and tyrosine was first obtained from cheese (in Greek, tyros means cheese.)
- Amino acids are classified as acidic, basic or neutral depending upon the relative number of amino and carboxyl groups in their molecule. 1. Equal number of amino and carboxyl groups makes it neutral 2. More number of amino than carboxyl groups makes it basic and 3. More carboxyl groups as compared to amino groups makes it acidic.
- The amino acids, which can be synthesized in the body, are known as nonessential amino acids.
- On the other hand, those which cannot be synthesized in the body and must be obtained through diet, are known as essential amino acids.
- Amino acids are usually colorless, crystalline solids. These are water-soluble, high melting solids and behave like salts rather than simple amines or carboxylic acids.
- This behavior is due to the presence of both acidic (carboxyl group) and basic (amino group) groups in the same molecule.
- In aqueous solution, the carboxyl group can lose a proton and amino group can accept a proton, giving rise to a dipolar ion known as zwitter ion. This is neutral but contains both positive and negative charges.
- In zwitter ionic form, amino acids show amphoteric behavior as they react both with acids and bases.
- Except glycine, all other naturally occurring α-amino acids are optically active, since the α-carbon atom is asymmetric.
Image: Amino Acids
Source: NCERT Text Books
- Proteins are the most abundant biomolecules of the living system. Chief sources of proteins are milk, cheese, pulses, peanuts, fish, meat, etc.
- They occur in every part of the body and form the fundamental basis of structure and functions of life.
- They are also required for growth and maintenance of body.
- The word protein is derived from Greek word, “proteios” which means primary or of prime importance.
- Proteins are polypeptides. [Peptide == a compound consisting of two or more amino acids linked in a chain].
- Proteins are linear chains of amino acids linked by peptide bonds.
- Each protein is a polymer of amino acids. [Monomer == a molecule that can be bonded to other identical molecules to form a polymer].
- Dietary proteins are the source of essential amino acids.
- Therefore, amino acids can be essential or non-essential. [Non-Essential Amino Acids == Amino Acids that our body can make]. [Essential Amino Acids == We get them through our diet/food].
- Collagen is the most abundant protein in animal world.
- Ribulose bisphosphate Carboxylase-Oxygenase (RuBisCO) is the most abundant protein in the whole of the biosphere.
- You have already read that proteins are the polymers of α-amino acids and they are connected to each other by peptide bond or peptide linkage.
- Chemically, peptide linkage is an amide [an organic compound containing the group -C(O)NH2] formed between –COOH group and –NH2
- The reaction between two molecules of similar or different amino acids, proceeds through the combination of the amino group of one molecule with the carboxyl group of the other.
- This results in the elimination of a water molecule and formation of a peptide bond –CO–NH–. The product of the reaction is called a dipeptide because it is made up of two amino acids.
- If a third amino acid combines to a dipeptide, the product is called a tripeptide.
- A tripeptide contains three amino acids linked by two peptide linkages.
- Similarly when four, five or six amino acids are linked, the respective products are known as tetrapeptide, pentapeptide or hexapeptide, respectively.
- When the number of such amino acids is more than ten, then the products are called polypeptides.
- A polypeptide with more than hundred amino acid residues, having molecular mass higher than 10,000u is called a protein.
- However, the distinction between a polypeptide and a protein is not very sharp.
- Polypeptides with fewer amino acids are likely to be called proteins if they ordinarily have a well-defined conformation of a protein such as insulin which contains 51 amino acids.
- Proteins can be classified into two types on the basis of their molecular shape: Fibrous Proteins and Globular proteins.
- When the polypeptide chains run parallel and are held together by hydrogen and disulphide bonds, then fibre– like structure is formed.
- Such proteins are generally insoluble in water. Some common examples are keratin (present in hair, wool, silk) and myosin (present in muscles), etc.
- This structure results when the chains of polypeptides coil around to give a spherical shape.
- These are usually soluble in water. Insulin and albumins are the common examples of globular proteins.
- Proteins may have one or more polypeptide chains. Each polypeptide in a protein has amino acids linked with each other in a specific sequence and it is this sequence of amino acids that is said to be the primary structure of that protein.
- Any change in this primary structure i.e., the sequence of amino acids creates a different protein.
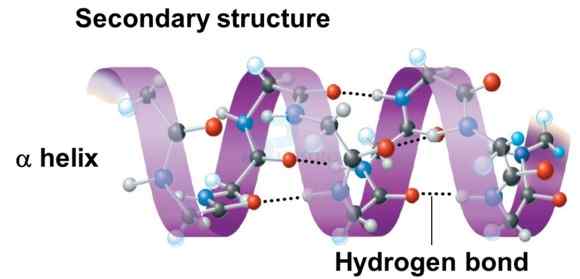
- The secondary structure of protein refers to the shape in which a long polypeptide chain can exist.
- Protein found in a biological system with a unique three-dimensional structure and biological activity is called a native protein.
- When a protein in its native form, is subjected to physical change like change in temperature or chemical change like change in pH, the hydrogen bonds are disturbed. Due to this, globules unfold and helix get uncoiled and protein loses its biological activity. This is called denaturation of protein.
- During denaturation 2° and 3° structures are destroyed but 1º structure remains intact. The coagulation of egg white on boiling is a common example of denaturation. Another example is curdling of milk which is caused due to the formation of lactic acid by the bacteria present in milk.
Image: Secondary structure
Source: NCERT Text Books
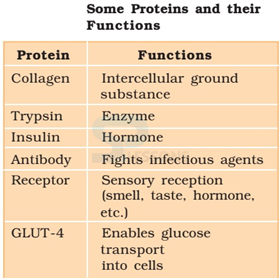
- Some transport nutrients across cell membrane
- some fight infectious organisms
- some are hormones
- some are enzymes, etc.
Image: Role of proteins
Source: NCERT Text Books
- Life is possible due to the coordination of various chemical reactions in living organisms. An example is the digestion of food, absorption of appropriate molecules and ultimately production of energy. This process involves a sequence of reactions and all these reactions occur in the body under very mild conditions. This occurs with the help of certain biocatalysts called enzymes. Catalyst == a substance that increases the rate of a chemical reaction without itself undergoing any permanent chemical change.
- Almost all the enzymes are globular proteins.
- Enzymes are very specific for a particular reaction and for a particular substrate.
- They are generally named after the compound or class of compounds upon which they work. For example, the enzyme that catalyses hydrolysis of maltose into glucose is named as maltase.
- Sometimes enzymes are also named after the reaction, where they are used. For example, the enzymes which catalyse the oxidation of one substrate with simultaneous reduction of another substrate are named as oxidoreductase The ending of the name of an enzyme is -ase.
- Almost all enzymes are proteins.
- There are some nucleic acids that behave like enzymes. These are called ribozymes.
- An enzyme like any protein has a primary structure, i.e., amino acid sequence of the protein.
- Enzyme catalysts differ from inorganic catalysts in many ways. Inorganic catalysts work efficiently at high temperatures and high pressures, while enzymes get damaged at high temperatures (say above 40°C).
- However, enzymes isolated from organisms who normally live under extremely high temperatures (e.g., hot vents and sulphur springs), are stable and retain their catalytic power even at high temperatures (up to 80°-90°C). Thermal stability is thus an important quality of such enzymes isolated from thermophilic organisms. Thermophile == a bacterium or other microorganism that grows best at high temperatures (above 45°C).
- The activity of an enzyme can be affected by a change in the conditions which can alter the structure of the protein. These include temperature, pH, change in substrate concentration or binding of specific chemicals that regulate its activity.
- Proteins are the polymers of about twenty different α-amino acids which are linked by peptide bonds.
- Ten amino acids are called essential amino acids because they cannot be synthesised by our body, hence must be provided through diet.
- Proteins perform various structural and dynamic functions in the organisms.
- Proteins which contain only α-amino acids are called simple proteins.
- The secondary or tertiary structure of proteins get disturbed on change of pH or temperature and they are not able to perform their functions. This is called denaturation of proteins.
- Enzymes are biocatalysts which speed up the reactions in biosystems. They are very specific and selective in their action and chemically all enzymes are proteins.
- In animal tissues, one notices the presence of all categories of compounds shown in Figure. These are called primary metabolites. However, when one analyses plant, fungal and microbial cells, one would see thousands of compounds other than these called primary metabolites, e.g. alkaloids, flavonoids, rubber, essential oils, antibiotics, colored pigments, scents, gums, spices. These are called secondary metabolites.
Image: Primary and Secondary Metabolities
Source: NCERT Text Books
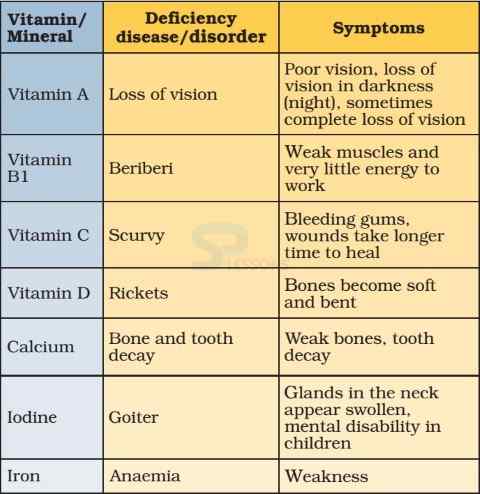
- Vitamins are organic compounds that are required in small amounts in our diet but their deficiency causes specific diseases.
- Most of the vitamins cannot be synthesized in our body but plants can synthesize almost all of them, so they are considered as essential food factors.
- However, the bacteria of the gut can produce some of the vitamins required by us.
- All the vitamins are generally available in our diet. Different vitamins belong to various chemical classes and it is difficult to define them on the basis of structure.
- They are generally regarded as organic compounds required in the diet in small amounts to perform specific biological functions for normal maintenance of optimum growth and health of the organism.
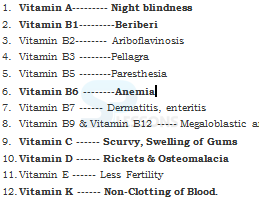
- Vitamins are designated by alphabets A, B, C, D, etc. Some of them are further named as sub-groups e.g. B1, B2, B6, B12, etc.
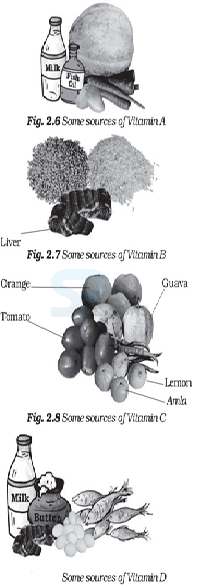
- Vitamin A keeps our skin and eyes healthy.
- Vitamin C helps body to fight against many diseases. Vitamin C gets easily destroyed by heat during cooking.
- Vitamin D helps our body to use calcium for bones and teeth.
- Excess of vitamins is also harmful and vitamin pills should not be taken without the advice of doctor.
- The term “Vitamine” was coined from the word vital + amine since the earlier identified compounds had amino groups.
- Later work showed that most of them did not contain amino groups, so the letter ‘e’ was dropped and the term vitamin is used these days.
- Vitamins are classified into two groups depending upon their solubility in water or fat. Fat soluble vitamins
- Vitamins which are soluble in fat and oils but insoluble in water are kept in this group. These are vitamins A, D, E and K. They are stored in liver and adipose (fat storing) tissues.
- B group vitamins and vitamin C are soluble in water so they are grouped together.
- Water soluble vitamins must be supplied regularly in diet because they are readily excreted in urine and cannot be stored (except vitamin B12) in our body.
- A person may be getting enough food to eat, but sometimes the food may not contain a particular nutrient. If this continues over a long period of time, the person may suffer from its deficiency.
- Deficiency of one or more nutrients can cause diseases or disorders in our body. Diseases that occur due to lack of nutrients over a long period are called deficiency diseases.
Image: Vitamin Deficiency Diseases
Source: NCERT Text Books
- Micronutrients, as opposed to macronutrients (protein, carbohydrates and fat), are comprised of vitamins and minerals which are required in small quantities to ensure normal metabolism, growth and physical well
- These are essential organic nutrients, most of which are not made in the body, or only in insufficient amounts, and are mainly obtained through food.
- When their intake is inadequate, vitamin deficiency disorders are the consequence.
- Although vitamins are only present and required in minute quantities, compared to the macronutrients, they are as vital to health and need to be considered when determining nutrition security.
- Each of the 13 vitamins known today have specific functions in the body: vitamin A, provitamin A (Beta‐carotene), vitamin B1, vitamin B2, vitamin B6, vitamin B12, biotin, vitamin C, vitamin D, vitamin E, folic acid, vitamin K, niacin and pantothenic acid.
- These are inorganic nutrients that also play a key role in ensuring health and well
- They include the trace elements copper, iodine, iron, manganese, selenium and zinc together with the macro elements calcium, magnesium, potassium and sodium.
- As with vitamins, minerals they are found in small quantities within the body and they are obtained from a wide variety of foods.
- No single food contains all of the vitamins and minerals we need and, therefore, a balanced and varied diet is necessary for an adequate intake.
- Of course, we already know a huge amount about how these work, and the importance they have in normal human growth and development.
- Based on this, an Expert Panel of nutritionist, NGOs and development agencies indentified five micronutrients such as those below in their priority group:
- This vital micronutrient is found in a range of different foods including carrots, spinach, broccoli, milk, egg, liver and fish.
- It plays an essential role in vision (lack of Vitamin A is a common cause of blindness), reproduction and growth, and the functioning of a healthy immune system (it plays a key role in the development of white blood cells).
- Worldwide about 5 million children under the age of five are affected by xerophthalmia, a serious eye disorder caused by vitamin A deficiency.
- These children are at risk of becoming blind and are more likely to die of common childhood diseases.
- This is a generic term for a group of B vitamins including folic acid and naturally occurring
- Folic acid is a synthetic folate compound used in vitamin supplements and fortified food because of its increased stability.
- Folates are found in egg, dairy products, asparagus, orange juice, dark green leafy vegetables, beans and brown bread.
- They play a key role in the metabolism of amino acids and the production of proteins, the synthesis of nucleic acid (the molecules that carry genetic information in the cells), and the formation of blood cells.
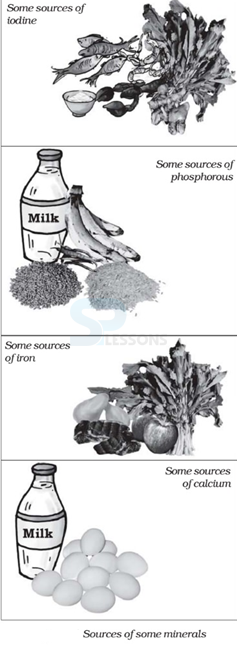
- Seaweed and fish are rich sources but in many countries the addition of iodine (known as iodization) to salt is an important source.
- Iodine is one of the most important elements required by the developing foetus due to its effect on brain development.
- Iodine also serves a number of other important functions especially in the production of hormones.
- Goitre is a visible sign of severe iodine deficiency.
- Iron has a number of key functions within the body. It acts as a carrier for oxygen from the lungs to the body’s tissues – it does so in the form of hemoglobin – and it also integral to the working of various tissues through the role that it plays in enzymatic reactions.
- Iron deficiency ultimately leads to iron deficiency anemia, the most common cause of anemia, a condition in which the blood lacks healthy red bloods cells required to carry oxygen, and which results in morbidity and death.
- Iron deficiency is the most widespread health problem in the world, impairing normal mental development in 40‐60% of infants in the developing world.
- Iron‐rich foods include lentils, red meat, poultry, fish, lentils, leaf vegetables and chick
- Found in a range of foodstuffs including liver, eggs, nuts, cereals and seafood.
- The absence of zinc is associated with a number of conditions including, short stature, anemia, impaired healing of wounds, poor gonadal function, and impaired cognitive and motor function.
- It can also lead to appetite disorders, as well as contributing to the increased severity and incidence of diarrhea and pneumonia.
- The most important effect of zinc deficiency is its impact on children’s resistance to infectious diseases including the risk of infection, the recurrence of infections and the severity of infection. This is well document in the case of diarrhoea. Zinc nutrition is therefore an important determinant of mortality in children.
Image: Food Source of Vitamins
Source: NCERT Text Books
Image: Source of Vitamin-D
Source: NCERT Text Books
Dietry Fibers- Dietary fibres are also known as roughage. Roughage is mainly provided by plant products in our foods.
- Whole grains and pulses, potatoes, fresh fruits and vegetables are main sources of roughage.
- Roughage does not provide any nutrient to our body, but is an essential component of our food and adds to its bulk. This helps our body get rid of undigested food.
- Fat is one of the three main macronutrients: fat, carbohydrate, and protein.
- Fat is a major source of energy and helps your body absorb vitamins.
- Fat has the most calories compared to any other nutrient. Controlling fat intake is one of the most important steps in losing or maintaining weight and preventing or delaying type 2 diabetes.
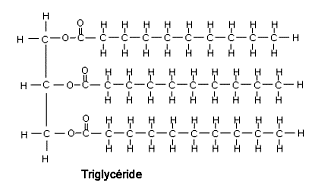
- Fats, also known as triglycerides, are esters of three fatty acid chains and the alcohol glycerol.
- Fats are solids at room temperature. Oil refers to a fat with unsaturated fatty acid chains that is liquid at room temperature.
- Fats, like other lipids, are generally insoluble in water.
- A lipid is chemically defined as a substance that is insoluble in water and soluble in alcohol and chloroform.
- Lipids are an important component of living cells. Together with carbohydrates and proteins, lipids are the main constituents of plant and animal cells.
- Cholesterol and triglycerides are lipids. Lipid is not necessarily a triglyceride.
- Glycerol is a simple sugar alcohol compound. A triglyceride is an ester derived from glycerol and three fatty acids (tri + glyceride)
- Triglycerides are the main constituent of body fat in humans and animals, as well as vegetable fat.
Image: Triglyceride
Source: NCERT Text Books
- A fatty acid is a carboxylic acid with a long aliphatic chain [organic compounds in which carbon atoms form open chains], which is either saturated or unsaturated.
- Some fatty acids are called essential because they cannot be synthesized in the body from simpler constituents.
- There are two essential fatty acids (EFAs) in human nutrition: alpha-linolenic acid (an omega-3 fatty acid) and linoleic acid (an omega-6 fatty acid).
- Fats and other lipids are broken down in the body by enzymes called LIPASES
- Fats are made of long chains of carbon (C) atoms. Some carbon atoms are linked by single bonds (-C-C-) and others are linked by double bonds (-C=C-).
Image: Saturated and Unsaturated Acid
Source: NCERT Text Books
Saturated fat- A saturated fat is a fat in which the fatty acids all have single bonds.
- A saturated fat has the maximum number of hydrogens bonded to the carbons, and therefore is ‘saturated’ with hydrogen atoms.
- Most animal fats are saturated whereas the fats of plants and fish are generally unsaturated.
- Many experts recommend a diet low in saturated fat.
- Saturated fats are popular with manufacturers of processed foods because they are less vulnerable to rancidity and are, in general, more solid at room temperature than unsaturated fats.
- An unsaturated fat is a fat or fatty acid in which there is at least one double bond within the fatty acid chain.
- Where double bonds are formed, hydrogen atoms are eliminated.
- In cellular metabolism, unsaturated fat molecules contain somewhat less energy (i.e., fewer calories) than an equivalent amount of saturated fat.
- The greater the degree of unsaturation in a fatty acid (i.e., the more double bonds in the fatty acid) the more vulnerable it is to rancidity [lipid oxidation][rusting of fats].
- Antioxidants can protect unsaturated fat from lipid oxidation.
- The main types of “healthy” fats are monounsaturated, polyunsaturated, alpha-linolenic acid (an omega-3 fatty acid) and linoleic acid (an omega-6 fatty acid).
- The fat is termed “monounsaturated” if there is one double bond, and “polyunsaturated” if there are two or more double bonds.
- Omega-3 and Omega-6 fatty acids are heart healthy fats and can help in lowering high triglyceride values in blood. They are found in fish, soybean products, Walnuts etc.
- Both of these fatty acids are needed for growth and repair, but can also be used to make other fatty acids.
- The omega-3 and omega-6 are fatty acids are both polyunsaturated. The difference is in where the first of the double bonds occurs.
- Both omega-3 (ω-3) and omega-6 (ω-6) fatty acids are important components of cell membranes.
- There is increasing support for omega-3 fatty acids in protecting against fatal heart disease and it is known that they have anti-inflammatory effects.
- There is also growing interest in the role of omega-3 fatty acids in the prevention of diabetes and certain types of cancer.
- Monounsaturated and polyunsaturated fat are considered “heart healthy” and can help with improving cholesterol when used in place of unhealthy fats.
- Some sources of these fats include almonds, cashews, pecans, peanuts, pine nuts, pumpkin, sesame seeds, sunflower seeds, Olive oil and olives, vegetable oils (such as sunflower, safflower, corn, soybean, and cottonseed).
Image: Good vs Bad Fats
Source: NCERT Text Books
- The main types of “unhealthy” fats are saturated and trans-fat.
- Saturated fats are primarily found in foods that come from animals, such as meat and dairy.
- Saturated fats are unhealthy because they increase LDL (“bad” cholesterol) levels in your body and increase your risk for heart disease.
- Many saturated fats are “solid” fats that you can see, such as the fat in meat. Other sources of saturated fats include high-fat cheeses, high-fat cuts of meat, butter, Ice cream, palm and coconut oils, etc..
- Trans fats, or trans-unsaturated fatty acids, trans fatty acids, are a type of unsaturated fats that are uncommon in nature.
- Trans fat is simply liquid oils turned into solid fats during food processing. There is also a small amount of trans fat that occurs naturally in some meat and dairy products, but those found in processed foods tend to be the most harmful to your health.
- Trans fats are worse than saturated fats. They increase LDL (“bad” cholesterol) and decreasing HDL (“healthy” cholesterol).
- Trans fatty acids are used as preservative in packaged food items. Foods containing trans-fat are usually labeled as “partially hydrogenated”.
- Partially hydrogenated oil is less likely to spoil, so foods made with it have a longer shelf life.
- Trans fats are easy to use, inexpensive to produce and last a long time. Trans fats give foods a desirable taste and texture.