Introduction
Introduction
Chemistry is the branch of science which deals with study of matter and various changes it undergoes. General Science Chemistry is an important section in the various competitive exams primarily the recruitment exams (Banking, SSC, Railways, RRB ALP, RRB Group D,etc.) in India. This General Science Chemistry Quick Guide presents the basics and key points of various Chemistry topics.
 Concepts
Concepts
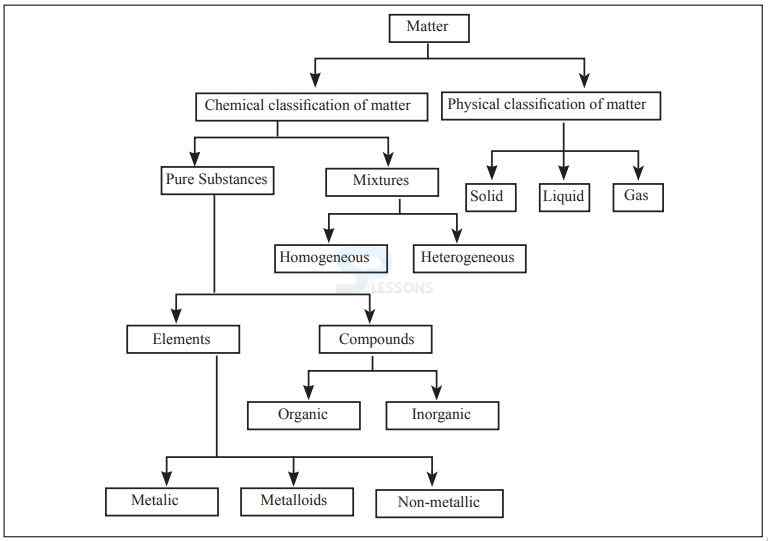
Classification of Matter:
Matter:
- It is defined as anything that occupies space and has mass.
- At a given temperature, an element is in one of the three states of matter- Solid, Liquid or Vapour (Gas).
- Solids possess definite shape and volume, eg. metals, brick, etc.
- They possess definite volume but no definite shape.
- They can flow, so they are called fluids, e.g. water, milk, mercury, oil,etc.
- Gases have neither a definite volume nor definite shape.
- They takes the volume and shape of the container. E.g.– air, oxygen, hydrogen, etc.
- Melting point of a substance is the temperature at which its solid form changes to a liquid.
- Boiling point is the temperature at which the liquid form of a substance changes to a gas.
- A physical change is a change in matter that involves no chemical reaction.
- The three types of physical changes are melting, evaporation and freezing.
- Chemical Change A change in which the identify of the original substance is changed and new substances are formed is called a chemical change for example souring of milk, burning of paper, rusting of iron etc.
Atom:
- An atom is the smallest unit of an element.
- An atom has a central nucleus.
- The nucleus carries a positive charge.
- Electrons revolves around the nucleus.
- Protons have a positive charge.
- Electrons have a negative charge.
- Neutrons have no charge.
- Everything in the universe is made of a combination of a few basic substances called elements.
- The element is the simplest form of matter composed of atoms having identical number of protons in each nucleus.
- A compound is a pure substance that contains atoms of two or more chemical elements in definite proportions that cannot be separated by physical means and are held together by chemical bonds.
Air and Water:
Air is colorless, odorless, tasteless, gaseous mixture, mainly contains nitrogen (approximately 78%) and oxygen (approximately 21%) with lesser amounts of argon, carbon dioxide, hydrogen, neon, helium, and other gases.
- Water consists of hydrogen and oxygen in the ratio of 2:1 by volume and 1:8 by mass. e.g. ([latex]H_2O[/latex])
- Hard water has bicarbonates, chlorides sulphates of Ca and Mg. This water is unfit for washing and use in industrial boilers.
- Heavy water is deuterium oxide ([latex]D_2O[/latex]), molecular mass = 20).
Substances and Chemical Compositions:
| Common Name | Chemical Name | Composition | Formula |
|---|---|---|---|
| Alum | Potash | Potassium, Sulphur, Aluminium, Hydrogen and Oxygen | [latex]K_2SO_4Al_2(SO_4)_3[/latex] |
| Bleaching Powder | Calcium hypochlorite | Calcium, Chlorine and Oxygen | CaCl(OCl) |
| Blue Vitriol | Copper sulphate | Copper, Sulphur and Oxygen | [latex]CuSO_4.5H_2O[/latex] |
| Caustic Potash | Potassium hydroxide | Potassium Hydrogen, and Oxygen | KOH |
| Chalk | Calcium carbonate | Calcium, Carbon and Oxygen | [latex]CaCO_3[/latex] |
| Caustic Soda | Sodium hydroxide | Sodium, Hydrogen and Oxygen | NaOH |
| Baking Soda | Sodium bicarbonate | Sodium, Hydrogen, Carbon and Oxygen | [latex]NaHCO_3[/latex] |
| Common Salt | Sodium chloride | Sodium and Chlorine | NaCl |
| Epsom Salt | Magnesium sulphate | Magnesium, Sulphur, and Oxygen | [latex]MgSO_4. 7H_2O[/latex] |
| Galena | Lead sulphide | Lead and Sulphur | PbS |
| Green Vitriol | Iron sulphate | Iron, Sulphur and Oxygen | [latex]FeSO_4. 7H_2O[/latex] |
| Glauber's salt Gypsum | Sodium sulphate Calcium Sulphate dihydrate | Sodium, Sulphur, Oxygen and hydrogen | [latex]Na_2SO_4.10H_2O CaSO_4.2H_2O[/latex] |
| Laughing gas | Nitrous oxide | Nitrogen and Oxygen | [latex]N_2O[/latex] |
| Lime water | Calcium hydroxide | Calcium, Hydrogen, and Oxygen | [latex]Ca(OH)_2[/latex] |
| Litharge | Lead monoxide | Lead and Oxygen | PbO |
| Plaster of Paris | Calcium sulphate hemihydrate | Calcium, Sulphur, Hydrogen and Oxygen | [latex]2CaSO_4.H_2O[/latex] |
| Quartz | Sodium silicate | Sodium, Silica and Oxygen | [latex]Na_2SiO_3[/latex] |
| Quick lime | Calcium oxide | Calcium and Oxygen | CaO |
| Red lead | Triplumbic | Lead and Oxygen | [latex]Pb_3O_4[/latex] |
| Sal ammoniac | Ammonium Chloride | Nitrogen, Hydrogen and chlorine | [latex]NH_4Cl[/latex] |
| Soda ash or washing soda | Sodium carbonate | Sodium, Carbon, Hydrogen and Oxygen | [latex]Na_2CO_3.10H_2O[/latex] |
| Soda bicarbonate | Sodium bicarbonate | Sodium hydrogen, Carbon and Oxygen | [latex]NaHCO_3[/latex] |
| White vitriol | Zinc sulphate | Zinc, Sulphur, Hydrogen and Oxygen | [latex]ZnSO_4.7H_2O[/latex] |
Metals and Non-Metals:
There are two types of elements metals and non- metals.
Metals:
- Elements which are hard, ductile, brittle, and malleable, possess lustre and conduct heat and electricity are termed metals.
- Except Mercury and gallium, all metals are solid.
- Non metals are electronegative elements which have a tendency to gain one or more electrons to form negative ions called anions.
- Non metals are non lustrous and bad conductors of heat and electricity.
- Minerals are naturally occurring chemical compounds of fixed composition and characteristics. e.g., silicates, oxides, sulphides, and carbonates, etc.
- Silver Nitrate ([latex]AgNO_3[/latex]) is called lunar caustic and is used to prepare the ink used during voting.
- Hydrogen Peroxide ([latex]H_2O_2[/latex]) is used as an oxidising agent, bleaching agent, as an insecticide and for washing old oil paintings.
- Ferric Oxide ([latex]Fe_2O_3[/latex])is used in jeweller's rouge.
- Silver Iodide ([latex]AgI[/latex])is used for artificial rain.
Fuels:
- The substance, which produce heat and light on combustion are called fuels.
- LPG (Liquified petroleum gas) is a mixture of hydrocarbons containing three or four carbon atoms, such as propane, butane and pentane.
- Coal is made up of carbon.
- The common varieties of coal are anthracite, bitumen; lignite and peat.
- Acids are chemical compounds that taste sour, turn blue litmus red, and often react with some metals to produce hydrogen gas.
- Acids- [latex]HNO_3[/latex], [latex]HNO_2[/latex], [latex]H_2SO_4[/latex], [latex]H_3PO_4[/latex], [latex]H_3PO_3[/latex], [latex]H_2CO_3[/latex], etc.
- Bases are chemical compounds that taste bitter, turn red litmus blue and feel slippery. Base: (NaOH), ([latex]Ca(OH)_2[/latex]), (KOH), (RbOH), etc.
- When aqueous (water) solutions of an acid and a base are combined, a neutralization reaction occurs.
- The pH of a solution measures the hydrogen ion concentration in that solution.
- Anything above pH 7 is alkaline, anything below pH 7 is considered acidic.
- Human blood pH should be slightly alkaline (7.35-7.45).
| Acid | Source |
|---|---|
| Citric acid | Lemon, orange, grapes |
| Maleic acid | Unripe apple |
| Tartaric acid | Tamarind |
| Acetic acid | Vinegar |
| Lactic acid | Milk |
| Hydrochloric acid | Stomach |
| Oxalic acid | Tomato |
| Acidic | Basic (Alkaline) |
|---|---|
| 1. Bathroom acid | 1. Milk of magnesia (Antacids) |
| 2. Vitamin C tablets (Ascorbic acid) | 2. Toothpaste |
| 3. Lemon juice | 3. Soap solution or detergent solution |
| 4. Orange juice | 4. Solution of washing soda. |
| 5. Tomato juice | 5. Slaked lime & white wash |
| 6. Vinegar | |
| 7. Fizzy drinks (Colas & Soda water) |
| Substance | pH Value |
|---|---|
| Sodium Hydroxide: Alkaline | 14.0 |
| Ammonia | 11.0 |
| Baking Soda | 8.3 |
| Human Blood | 7.35 to 7.45 |
| Pure Water: Neutral | 7.0 |
| Milk: Acid | 6.6 |
| Tomatoes | 4.5 |
| Wine and Beer | 4.0 |
| Apples | 3.0 |
| Vinegar | 2.2 |
| Lemon Juice | 2.0 |
| Battery Acid | 1.0 |
| Urine(Human) | 5.5 to 7.5 |
| Tears | 7.4 |
| Sea water | 8.5 |
| Milk (Cow) | 6.3 to 6.6 |
| Coffee | 5.0 |
| Tooth paste | 9.0 |
- Plastics consist of very long molecules, each composed of carbon atoms linked into chains.
- Polythene is composed of over 200000 carbon atoms.
- Polymers are large long chain like molecules formed by the chemical linking of many smaller molecules.
| Polymer | Use |
|---|---|
| Polythene | Packaging material, carry bags, bottles etc. |
| Polypropene | Bottles, Crates etc. |
| Polyvinyl chloride (PVC) | Pipes insulation |
| Nylon (Polyester) | Fibres, ropes etc |
| Teflon | Nonstick kitchen wares |
| Vinyl rubber | Rubber erasers |
| Polystyrene | Foam Thermocole |
| Poly (Styrene butadiene) | Rubber bubble gum |
| Bakelite | Electrical insulation buttons |
| Lexan | Bullet proof glass |
| Melamine | Crockery |
- Radioactivity is discovered by French physicist Henry de Becquerel in 1896, who observed that uranium mineral gave off invisible radiation.
- Radiations are of three kinds: Alpha, Beta and Gamma
- Alpha (α) Particle is positively charged helium atom that has very little penetrating power.
- Beta (β) Particles These are negatively charged light particles.
- Gamma (γ) Particles These are electromagnetic radiations of low wavelength, high frequency, and high energy.
- It is a process of plating one metal onto another by electrolysis, most commonly for decorative purposes or to prevent corrosion of a metal.
- Types of electroplating capsopper plating, silver plating, and chromium plating, etc.
Glass is a mixture of an alkali silicate with the silicate of a base, that is, silica, sodium silicate and calcium or lead silicate.
Type and Uses:
- Milky Glass is used to the melt glass.
- Flint Glass, used in lenses, prisms.
- Soda or Soft Glass is used for making bottles, window panes, etc.
- Potash Glass or Hard Glass is used for making beakers, flasks, funnel, etc.
- Crown Glass is used for optical apparatus.
- Crook's Glass is used for spectacles as it absorbs UV rays.
- Glass Laminates is used to make windows and screens of cars, trains and aircraft.
- Jena Glass is used for making laboratory bottles, for keeping acids and alkalies.
Soaps and Detergents: Soaps are the sodium or potassium salts of fatty acids.
Antibiotic: Medicinal compounds produced by moulds and bacteria, capable of destroying or preventing the growth of bacteria in animal systems. For example penicillin, chloramphenicol etc.
Antibody: Kinds of substances formed in the blood, tending to inhibit or destroy harmful pathogens, etc.
Antigen: Substance capable of stimulating formation of antibodies in a host. For example bacteria, virus etc.
Antipyretic: A substance used to lower body temperature.
Sulphadrugs: Alternatives of antibiotics, sulphanilamide, sulphadiazine, Sulpha gunamidine.
Antacids: Substances which neutralise the excess acid and raise the pH to appropriate level in stomach are called antacids.
Chloroform: A sweetish, colourless liquid. It is used as a solvent and anaesthetic.
Saccharin: A white crystalline solid which is 550 times sweeter than sugar, but does not have any food value. It is used by diabetic patients.
DDT: Dichloro diphenyl tricholoro ethane, a white powder used as an insecticide.
Branches of Science:
| Branch | Terminology |
|---|---|
| Adenology | Study of glands |
| Angiology | study of blood flow and lymphatic system |
| Arthrology | Study of joints |
| Barology | Study of gravitation |
| Bromatology | Study of food |
| Carpology | Study of fruits and seeds |
| Cetology | study of whales and dolphins |
| Cosmology | Study of the universe |
| Craniology | Study of the skull |
| Dactylography | The study of fingerprints |
| Demology | Study of human behaviour |
| Ecology | Study of environment |
| Endocrinology | Study of ductless glands |
| Entomology | Study of insects |
| Geology | Study of earth's crust |
| Hematology | Study of blood |
| Hepatology | Study of liver |
| Herpetology | Study of reptiles and amphibians |
| Hypnology | Study of sleep; study of hypnosis |
| Ichthyology | Study of fish |
| Irenology | The study of peace |
| Kalology | Study of beauty |
| Laryngology | Study of larynx |
| Mastology | Study of mammals or mammary glands or breast diseases |
| Meteorology | Study of weather |
| Myology | Study of muscles |
| Neonatology | Study of newborn babies |
| Nephrology | Study of the kidneys |
| Obstetrics | Study of midwifery |
| Odontology | Study of teeth |
| Oncology | Study of tumours |
| Pathology | Study of disease |
| Pharmacology | Study of drugs |
| Physiology | Study of processes of life |
| Pyretology | Study of fevers |
| Radiology | Study of X-rays and their medical applications. |
| Seismology | Study of earthquakes |
| Toxicology | Study of poisons |
| Urology | Study of urine; urinary tract |
| Virology | Study of virusesstudy of viruses |
| Xylology | Study of wood |
| Zoiatrics | Veterinary surgery |
| Zoology | Study of animals |